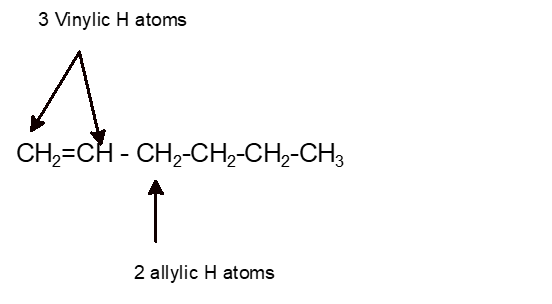
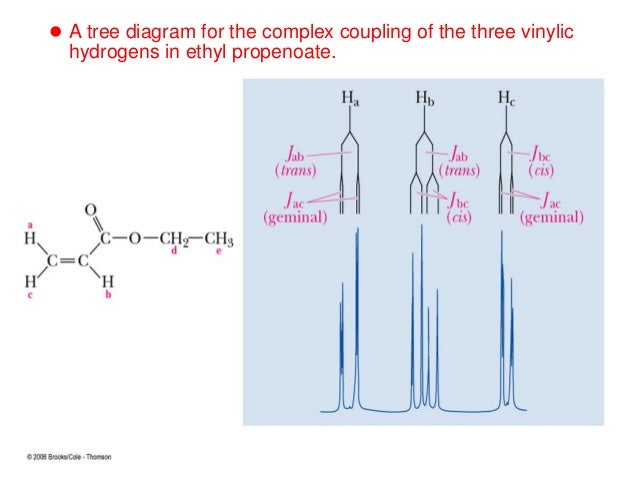
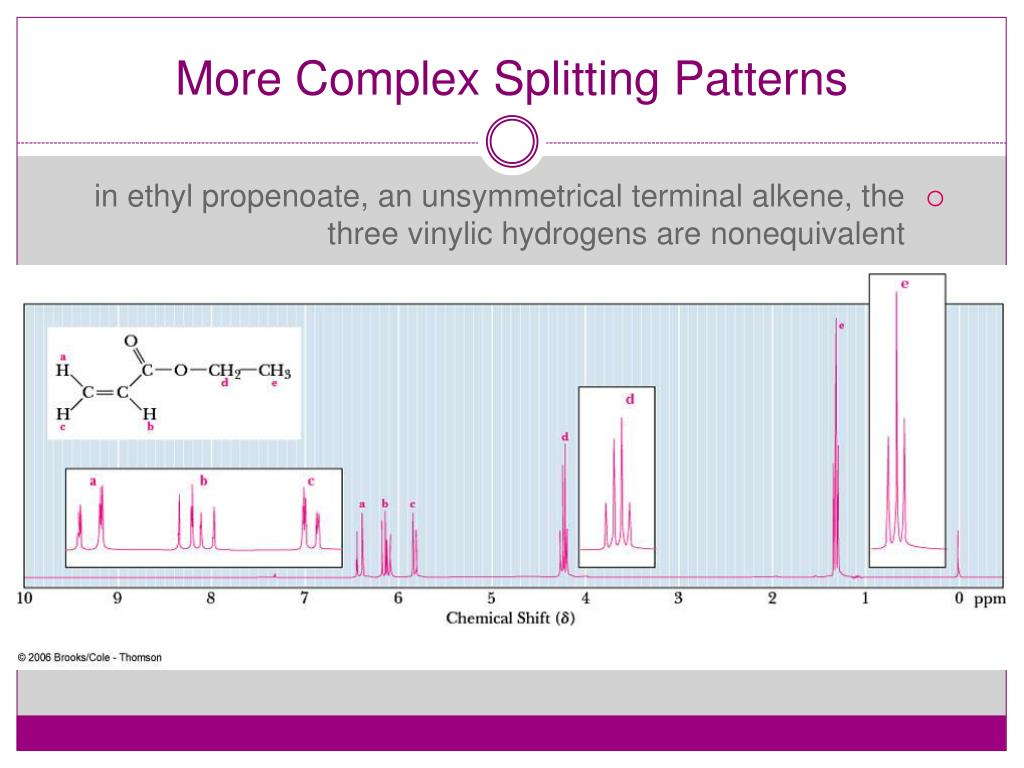
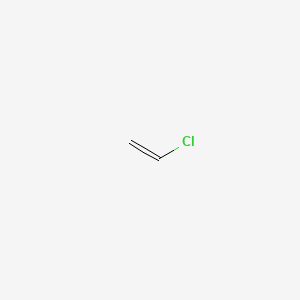
Three Vinylic Hydrogens

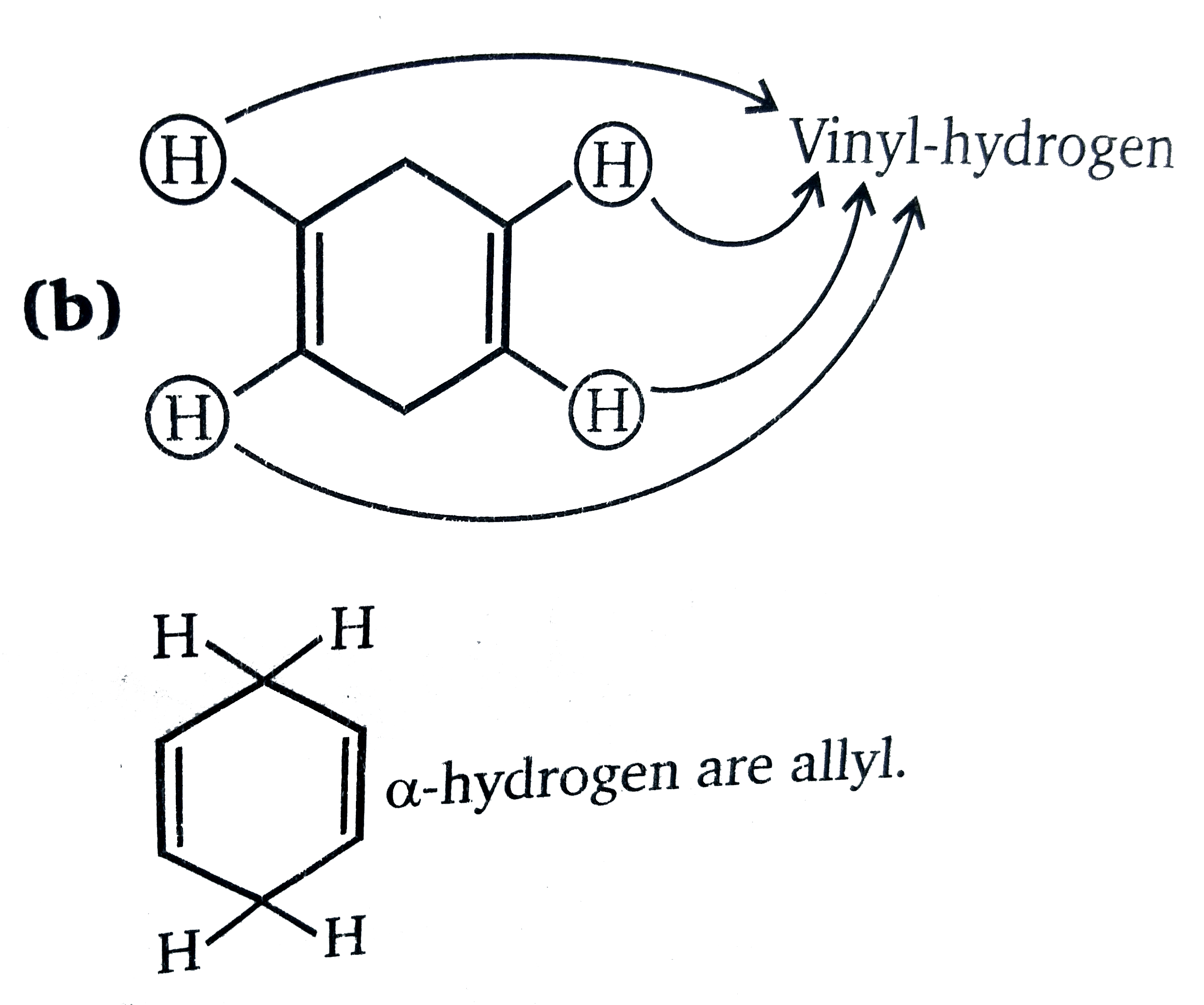
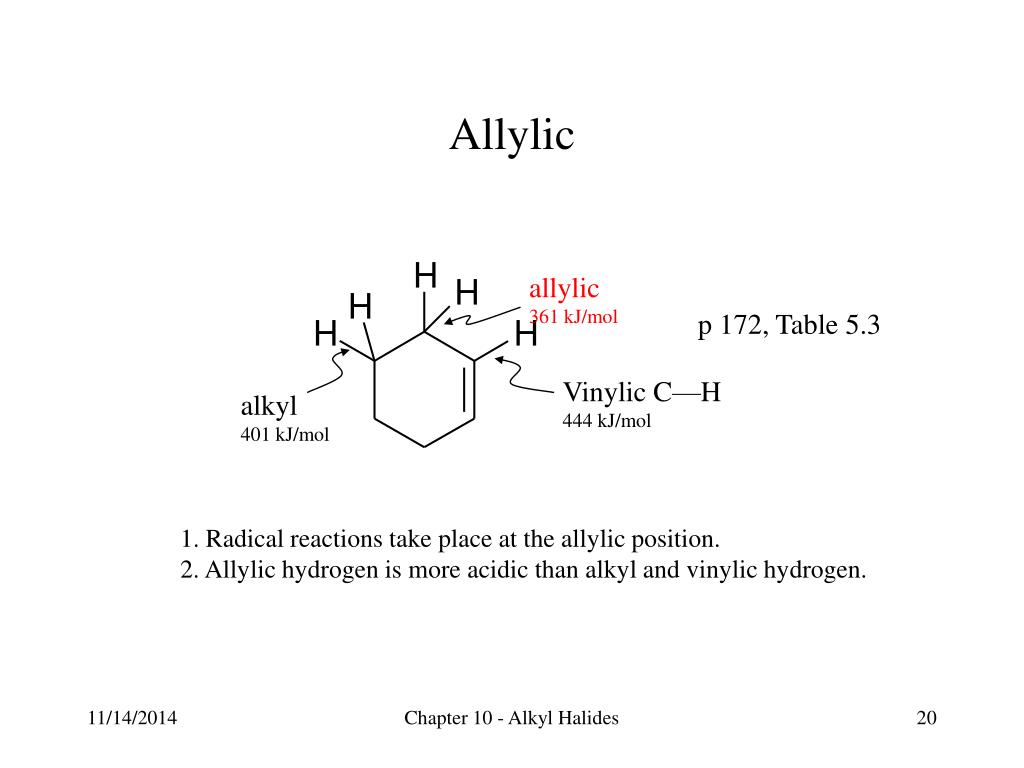
Benzylic position allylic position propargylic.
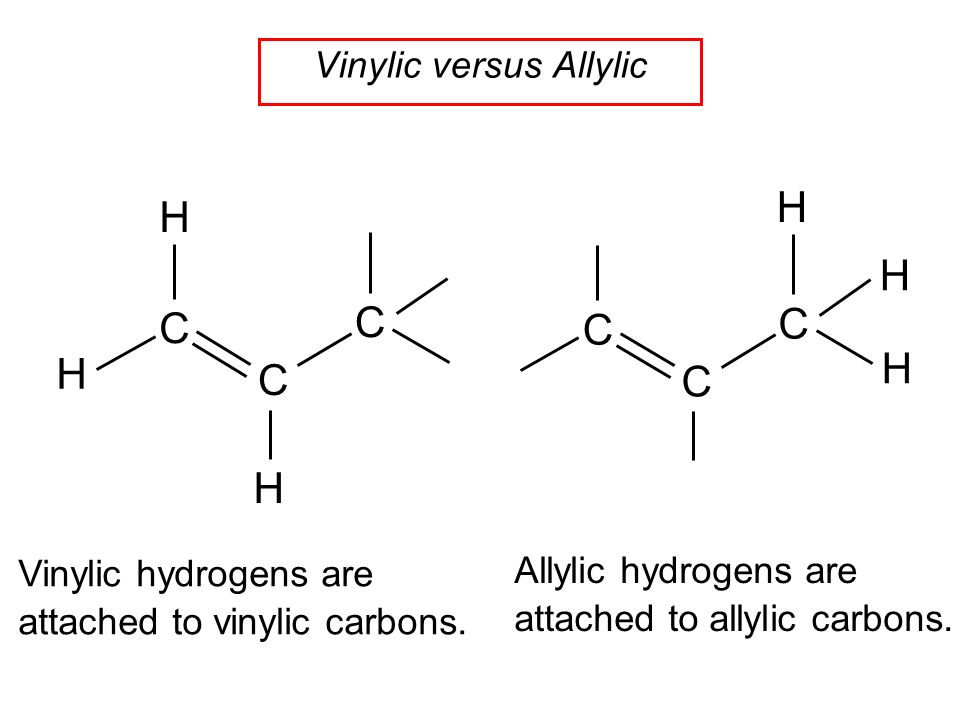
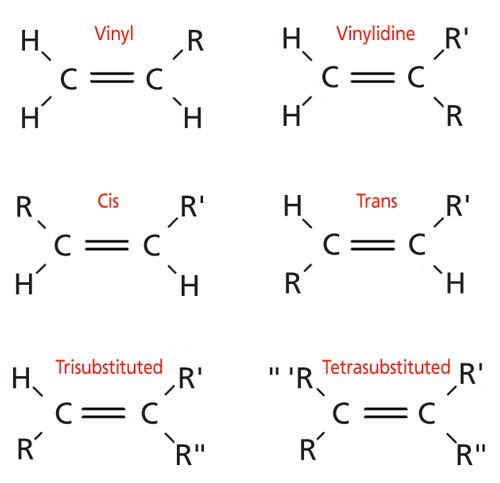
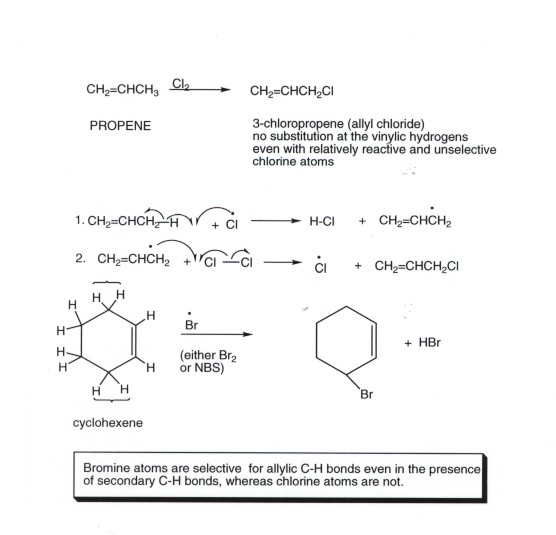
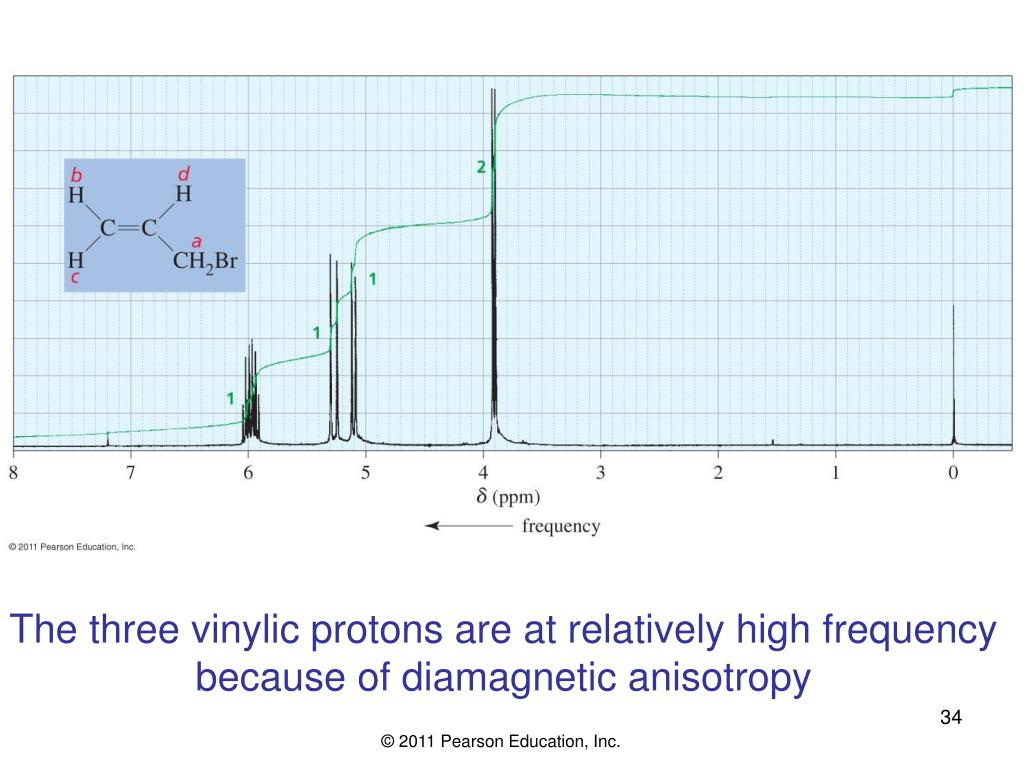
Three vinylic hydrogens. The libretexts libraries are powered by mindtouch and are supported by the department of education open textbook pilot project the uc davis office of the provost the uc davis library the california state university affordable learning solutions program and merlot. The vinylic hydrogens are shown in red. None of the other hydrogens are vinylic. Vinylic c h bond is lower.
Vinylic carbon can have either two double bonds in its sides or one double bond. However hydrogens of external alkynes resonate at lower frequency than vinylic hydrogens at appear at 2 3 ppm range. Number of hydrogen atoms. Give the structures of a hydrocarbon that has six carbon atoms and a three vinylic hydrogens and two allylic hydrogens b three vinylic hydrogens and no allylic hydrogens.
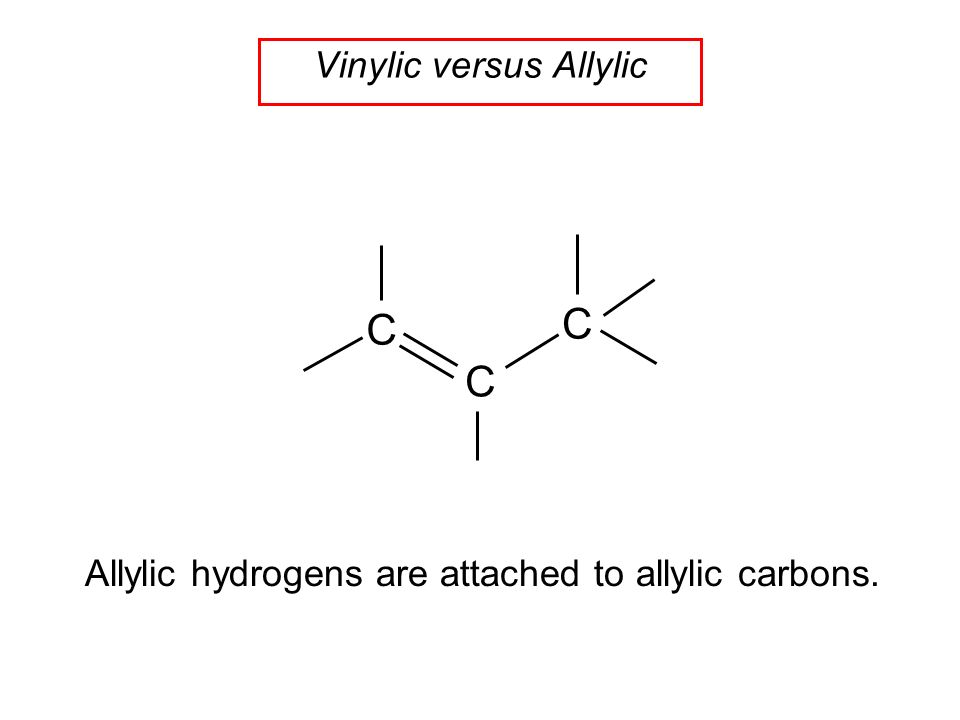
A hydrogen atom bonded to an sp 2 carbon of an alkene. C three vinylic hydrogens and ane allylic hydrogens. We also acknowledge previous national science foundation support under grant numbers 1246120 1525057 and 1413739. Allylic carbon only forms a single bond.
It forms at least one double bond. This puts the proton in a shielded environment and thus it feels a weaker magnetic. The reason is that unlike alkenes the induced magnetic field of the p electrons in the triple bond is opposite to the applied magnetic field.